Clinical Evidence
Traumeel compared with NSAIDs (mainly Diclofenac) for symptomatic treatment of epicondylitis
Reference:
Birnesser H, Oberbaum M, Klein P, Weiser M. The homeopathic preparation Traumeel S compared with NSAIDs for symptomatic treatment of epicondylitis. J Musculoskeletal Research. 2004; 8(2-3):119-128.52Study design
- Patients with diagnosed epicondylitis were treated with:
- Traumeel injection (local infiltration) n=86: 40 male, 43 female; mean age 48.6 years
- NSAIDs (unspecified, mainly diclofenac 51.9%) injection (systemic, mainly intramuscular) n=77: 40 male, 36 female; mean age 45.8 years.
- Other treatments were allowed, e.g. oral analgesics or physiotherapy, but while Traumeel patients were allowed further injections, they were not allowed oral NSAIDs: 41.6% of the NSAID group received oral Diclofenac.
- Assessments conducted at weeks 1 and 2.
Outcome measures
- Pain: local pressure pain, pain with movement, pain at rest. 5-point scale: 0=no pain, 1=light, 2=moderate, 3=strong, 4=severe.
- Mobility: extensional joint mobility, torsional joint mobility. 4-point scale: 1=normal, 2=lightly impaired, 3=moderately impaired, 4=heavily impaired.
- Global assessment of efficacy: time to first improvement, outcome of therapy (very successful, successful, moderate, unsuccessful), compliance (very high, high, moderate, low).
Figure: Mean difference with 97.5% confidence interval between symptom scores after two weeks for patients treated with NSAIDs (mainly Diclofenac) (n=77) and Traumeel Injection Solution (n=86).
Results
- Both treatments showed similar improvements in all five variables in the first week with no significant differences in time to onset of action.
- Traumeel Injection Solution showed markedly greater improvements in the variables pain at rest (p<0.01), change in extensional joint mobility (p<.05) and change in torsional joint mobility (p<0.01) compared with diclofenac and other nsaids particularly in the second week of treatment (p values from non-inferiority analysis at end of week 2).
- Although the study was designed to assess non-inferiority, the analysis showed Traumeel Injection Solution to be equivalent to Diclofenac and other NSAIDs on all variables and trended towards superiority on the variables pain at rest, extensional joint mobility and torsional joint mobility (see Figure).
- In global assessment, treatment was judged "very good" or "good" in 71% of Traumeel patients compared with 44% of NSAID patients (p=0.013).
- Compliance was reported as "very high" or "high" in 92% of Traumeel patients compared with 81% of NSAID patients (p=0.11).
Conclusion
- Traumeel Injection Solution was at least equivalent to NSAID therapy (mainly Diclofenac) in reducing pain and improving mobility in the early treatment of epicondylitis.
Drug surveillance study for Traumeel Injection Solution
Reference:
Zenner S, Metelmann H. Application possibilities of Traumeel S Injection Solution: results of a multicenter drug monitoring trial conducted on 3,241 patients. Biol Ther. 1992; X (4):301-310Study design
- 348 physicians completed surveys for patients in their care receiving Traumeel Injection Solution: 3,241 patients: 49.1% male, 50.5% female; mean age 47.5 years.
- The most frequent complaints were forms of degenerative joint disease (primarily of the knee and hip), followed in descending order of frequency by myogelosis and sprains. Periathropatia humeroscapularis, epicondylitis and tendovaginitis were also treated.
- Duration of symptoms was <1 week for 33.9% of patients, between 1 week and 1 month for 31.0%, and over 1 month for 33.7%.
- Traumeel was the only treatment for 19.2% of patients: 33.3% received non-pharmacological therapy (e.g. application of heat or cold, massage), 14.9% received additional medical therapy (which could include other preparations of Traumeel), and 31.1% combined additional medical and non-pharmacological therapy.
- Frequency of application: daily 15.2%, 3 times a week 27.7%, twice weekly 40.1%, once weekly 13.6%.
- Manner of application: intramuscular 24.0%, subcutaneous 17.8%, i.m. around the joint 14.6%, intra-articular 10.6%, peritendineal 7.0%, intravenous 4.3%, intracutaneous 2.8%, other 18.6%.
- Duration of treatment: <1 week 15.9%, 1 week to 1 month 62.7%, 1-3 months 15.2%, 3-6 months 3.2%,>6 months 2.1%.
Outcome measures
- Physician-rated therapy outcome: very good, good, satisfactory, unsuccessful, worsened.
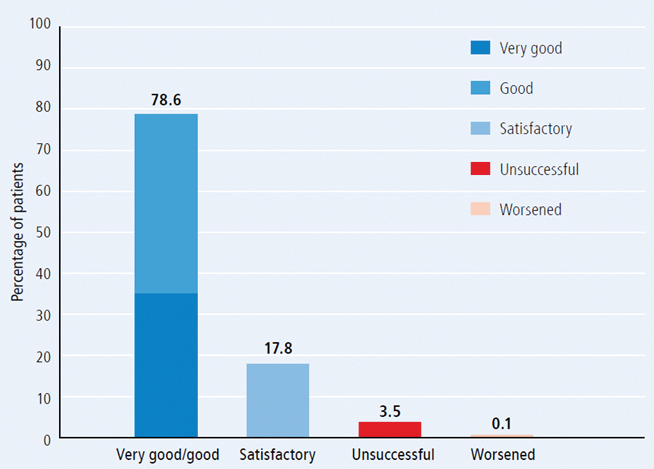
Figure: Results of therapy among patients treated with Traumeel injection (n=3,421).
Results
- The overall therapeutic results were graded as "very good" or "good" in 78.6% of cases. Treatment was "unsuccessful" in only 3.5% of cases and only five cases (0.1%) were reported as "worsening" (see Figure 13).
- Results were rated as "good" or "very good" in 95.0% of patients with sprains, 86.9% tendovaginitis, 80.1% myogelosis, 78.6% epicondylitis, 74.8% periathropathia humeroscapularis and 59.5% degenerative joint disease.
- Ratings appear higher when Traumeel was administered without concomitant therapies: 85.2% "good" or "very good" for monotherapy, 79.6% additional non-medical therapy, 82.8% additional medical therapy, and 71.7% additional medical and non-medical therapies.
- The fraction of "good" or "very good" results was greater with shorter administration intervals between injections than for applications with longer time periods between injections; e.g. daily application resulted as "good" and "very good" comments in 90.1%, weekly application only in 68.2%.
- Traumeel was well tolerated.
Conclusion
- Traumeel Injection Solution is effective for therapy of post-traumatic conditions (sprains), as well as inflammatory and degenerative processes affecting the musculoskeletal system.
Pilot Study: Comparative Outcomes of Cervical and Lumbar Spine Facet Injections
Reference:
Cecil Graham, MD, Reiner Kremer, NMD, MPH, Russell Erhardt, DC, AZ Pain Centers, Peoria, Arizona. Comparative Outcomes of Cervical and Lumbar Spine Facet Injections using Betamethasone Sodium Phosphate and Betamethasone Acetate Injectable Suspension vs. Traumeel (Arnica Montana, et al Injectable Solution) for Pain and Dysfunction in Chronic Pain States. Poster presentation at the AAPM conference 2010.54Study design
- 68 chronic pain patients were asked to complete the Pain Disability Questionnaire (PDQ), a functional outcomes measurement tool, at the onset and prior to each facet procedure.
- The PDQ tracks 13 subjective responses to pain on emotional state, financial impact, and activities of daily living (ADL).
- Data was collected over a four month period on patients with a minimum of three procedures for the same complaint.
- A comparison was made between patients receiving a steroid solution containing Betamethasone (n=27) and a non-steroidal solution containing Traumeel (n=41). The primary efficacy outcome was based on pain relief reports, and the secondary efficacy outcome was the emotional state changes.
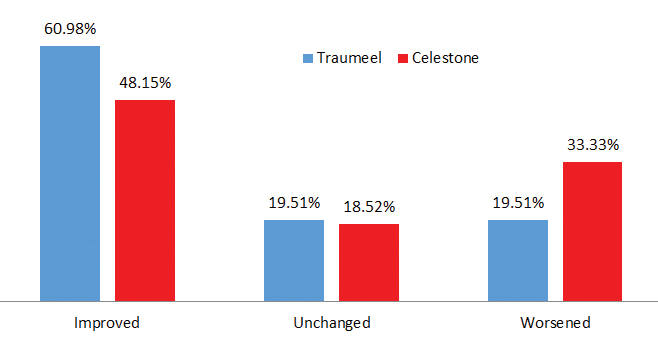
Figure: Based on these preliminary results it appears that cervical and lumbar spine facet injections with Traumeel Injection Solution may lead to beneficial results.
Results
- Preliminary results indicate that 25 patients (60.98%) treated with the Traumeel Injection Solution demonstrated significant improvement, while 8 patients (19.51%) worsened, and 8 (19.51%) stayed the same.
- Patients treated with Celestone-Betamethasone had poorer outcomes: 13 patients (48.15%) demonstrated improvement; 9 patients (33.33%) worsened, and 5 (18.52%) stayed the same.
Conclusions
- These preliminary results indicate that Traumeel Injection Solution improves pain and functional scores at a higher rate when compared with Celestone (Betamethasone) in patients with chronic back pain receiving cervical and lumbar facet injections.
- The mechanism of action of Traumeel Injection Solution includes the regulation rather than ablation of the cyclooxygenase pathway, therefore, pain is still addressed and normal physiologic mechanisms are otherwise unaffected.
Treatment of Lumbar and Cervical Pain
Reference:
J. Timmermann & Frase, W. (1997): Lumbar and Cervical Pain: Intervertebral Treatment with a Homeopathic Medication. Biomedical Therapy. Vol XV. No. 3. 1997.106Study design
Over a course of three years patients that suffered from lumbar or cervical pain in different clinical stages were assessed by clinicians and either corticosteroids or Traumeel Injection Solution applied as CT-guided i.a. injections.Lumbar Pain Syndrome:
- Patients with lumbar pain syndromes that were resistant to therapy for longer than six weeks and who previously had been treated by an orthopedic specialist, assessment of the status of the lumbar spine was carried out using computed tomography
- After assessing the presence of intravertebral arthrosis and making adequately sure that no disc pathologies were present, these patients received i.a. therapy for 5 days
- In the absence of risk factors (e.g. diabetes, osteoporosis or pronounced lumbalgia) corticosteroids were administered i.a. over a period of five days
- In cases of slightly restricted bodily functioning, i.a. injections of 40 mg corticosteroid were administered on both sides on days 1, 3, and 5, while one ampule of Traumeel Injection Solution was injected to each side on days 2 and 4
- In higher risk groups, with active metabolic disorders or pronounced osteoporosis, only Traumeel Injection Solution was administered i.a. on all five days
- In total, 236 patients were treated; 76 of them could be assessed in follow-up visits after four and sixteen weeks
Cervical Pain Syndrome:
- 24 patients were treated for cervical complaints and received injections around the joint into the nerve roots of the cervical vertebral joints with one ampule of Traumeel Injection Solution on each side.
- Therapy was administered on days 1, 3, and 5.
- The joints C4/5, C5/6, and C6/7 were targeted for injection.
Results
- After four weeks, positive results were observed in the 76 patients that were monitored
- In most cases, subjective reports on improvement coincided with observation of improved back function
- The positive development after sixteen weeks were surprising as successful results continued to be noted in the majority of patients
- The authors pointed out that combining corticosteroids with Traumeel in graduated amounts allows to individualize the treatment of patients with back pain
- Especially in patients with pronounced metabolic disorders, targeted injections with Traumeel Injection Solution allow to treat back pain with reduced amounts of or even without corticosteroids.