Rx Medications in Development and Testing
The following information is provided by the developers of Infeperium, and is exclusive to Medicus LLC for their negotiations, for research purposes only, with representatives of the Veterans Administration, and certain pre-approved European and US Health providers and Bio/Pharma Companies solely! Infeperium is a non-FDA approved treatment and is not available in the United States.Infeperium an "Immunomodulator" or Biological Response Modifier (BRM)
Introduction
Diseases overpower the immune system - for some such assaults there has been no proven effective treatment. Autoimmune diseases are a family of more than 80 chronic, and often disabling, illnesses that develop when defects in the immune system lead the body to attack its own cells, tissues, and organs. Some common autoimmune diseases include Multiple Sclerosis, Type 1 Diabetes, Diabetic Wounds, Chronic Wounds, Chronic Pain, Rheumatoid Arthritis, Chronic Plaque Psoriasis, and Lupus. It is estimated that over 40% of all diseases are autoimmune.In addition, Chronic Lyme disease, which is not technically an autoimmune disease, but can mimic some of the common symptoms of Systemic Lupus Erythematosus; various forms of Therapy Resistant Cancers; Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), which is an acquired immune-mediated inflammatory disorder of the peripheral nervous system; and Alzheimer's disease and dementia, are all looking for a cure. We believe Infeperium if used as an individual treatment or in conjunction with other available treatments, may lead to remission, and over time may may be considered a cure.
The traditional response to these diseases has been pharmaceuticals & chemicals with toxins and negative side effects and potential habit forming Narcotics. You only have to listen to the pharmaceutical companies TV ads to know that the side effects are potentially as harmful as the disease.
Mission
To take the qualities and characteristics of Infeperium as a biological response modifier (BRM) and anti-inflammatory agent and focus its therapeutic and prophylactic benefits on chronic diseases and conditions that have no available treatment or present treatments are ineffective.Description of Product
Infeperium, the natural product, and Infeperium, the synthesized product, are both a refinement of a USDA approved immunomodulator for treatment of domestic animals. An Immunotherapeutic biological response modifier (BRM) that functions in part through affecting the cytokine profile. They function in both a prophylactic and therapeutic manner. BRM's modulate and regulate the body's immune system and response without toxins or negative events or forming habits. BRM's are a substance that can improve the body's natural response to infection and disease. BRM's, a new drug class and an FDA category of their own, are medications which are based on compounds that are made by living cells.At this time we know of no other therapeutic compound that modulates both the Innate and Adaptive immunity. It is our opinion that the peptide, Infeperium, is a major breakthrough!
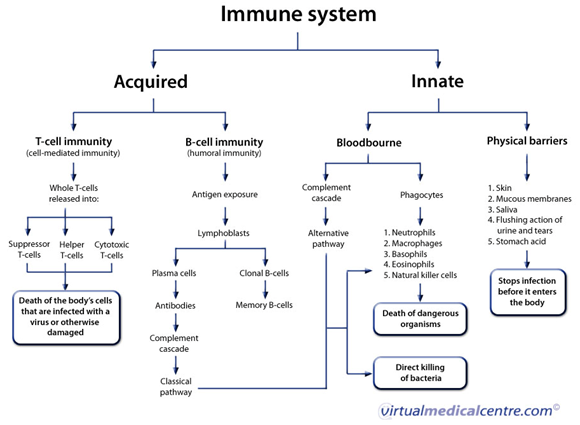
Innate immunity refers to nonspecific defense mechanisms that come into play immediately or within hours of an antigen's appearance in the body. These mechanisms include physical barriers such as skin, chemicals in the blood, and immune system cells that attack foreign cells in the body.Adaptive immunity refers to antigen-specific immune response. The adaptive immune response is more complex than the innate. The antigen first must be processed and recognized. Once an antigen has been recognized, the adaptive immune system creates an army of immune cells specifically designed to attack that antigen.
History
Infeperium started into development for commercial use for humans in February of 2015. Before then, it had been in development and testing, under Compassionate Use Waivers, for over 10 years. The patient success of these treatments, in cases ranging from chronic plaque psoriasis, lyme disease, diabetic wounds, and other non-treatable diseases led to the commercial release in several countries outside of the United States. The genesis for Infeperium is a USDA approved immunomodulator for domestic animals that has the same active ingredient as Infeperium.In a recent test our Dr. Ken Willeford in association with TriMetis, designed a salmonellosis challenge model to assess the efficacy of Infeperium along with the synthesized peptides. On Day 6 of the study the test observed 100% mortality in the Control and Peptide Treated groups but observed a significant 56% survival rate in the Infeperium treated group. Ken states, "The observation that Infeperium provides immunomodulatory effects is FANTASTIC and gives us something more to build on. It confirms our rationale for its use in human conditions and gives us a center piece to construct a research platform around."
Infeperium is approved for human use in research for homeopathic treatment in Mexico. We expect, when fully approved and available on the market, that treatment with Infeperium of Traditional Therapies for Medical Conditons can be done more effective and at a fraction of their cost. This includes, but is not limited to:
- Neuropathy
- Diabetic Wound Care
- Diabetes related surgical amputations
- Psoriasis
- Multiple Sclerosis
- Brain Cancer
- Lung Cancer
- Prostate Cancer
- Cervical Cancer
- Pancreatic Cancer
- Fibromyalgia
- Epstein-Barr Virus (EBV)
- Crohn's Disease
- Irritable Bowel Syndrome
- Ulcerative Colitis
- And many more...
To see a Cost Study of Traditional Therapies for Medical Condition that can be treated with Infeperium click here.
Infeperium is presently made according to the most rigid standards of Current Good Manufacturing Practices (cGMP).The active ingredient in Infeperium has already met the rigorous standards for Veterinary use and it is approved for animal prescription under the USDA!
We believe Infeperium to be a new category of drug that may be characterized as a Biological Response Modifier and an "Immunomodulator". We believe that many disease pathologies that affect individuals are the result of an over-active immune system. Specifically, when a viral agent begins to adversely affect an individual's cells, the immune system frequently becomes overactive, which destroys the viral agent but also injures surrounding healthy cell structures. We believe other disease pathologies suppress an individual's immune system, which allows other diseases and agents to kill healthy cells. Although research is always ongoing, the product in its present form is complete. Research has concluded that Infeperium regulates an individual's immune system to prevent it from both over-reacting and under-reacting to a viral invasion of an individual's body systems. We believe that Infeperium contains a number of unique peptide or lipopeptide molecules which may neutralize viral pathogens and their inhibitory properties by activation of a cytokine system. This, in turn, will enhance an individual's cell mediated immunity and augment the individual's humoral immune system possibly by eliminating negative inhibitory cytokine factors and pathogenic freefloating organisms, while simultaneously sparing normal and healthy cells. Our objective is the continued development of Infeperium and its cognates and variants for treatment of multiple medical conditions.
- Infeperium has been found to rapidly affect the expression of proteins involved in transcription/translation/and post-translational events (folding via chaperones and disulfide linkages). The key to the effectiveness of Infeperium is that it is not a pathogen or a foreign agent used to provoke an immune response, but is a small molecular weight protein found in healthy mammals that is an immune modulating signaling molecule.
- Infeperium regulates Interferon Inducible Proteins - regulation of macrophage, microbacteriocidal activity and viral activity, identification and regulated apoptosis of mutated cellular activity.
- Infeperium upregulates the IL-10 shut off switch for chronic inflammation, while maintaining acute inflammation pathways.
- Infeperium restores proper cell and chemical messaging pathways
- Infeperium protects from oxidative stress by proper balance of Reactive Oxygen Species (ROS) and Wound Repair through specified angiogenesis.
- Infeperium restores proper and amplifies Cytokine cascades.
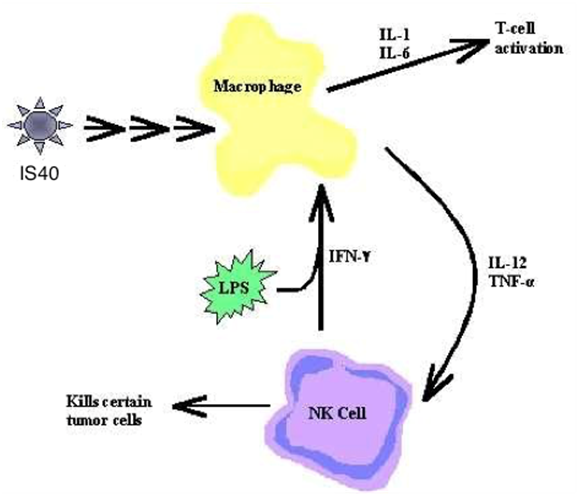
The following illustration demonstrates the immunomodulating cascade and the enhancement of Macrophage, Cytokine activity, TNF-a, and NK cells, as well as the up-regulating and down-regulating of IL-6, IL-10, IL-12, GM-CSF and IFN-a.
Click here to learn more about the Protocols and Procedures for the Administration of Infeperium
A Major Breakthrough in Immunotherapy
We have a number of breakthrough immunomodulators and biological response modifiers that have dramatic effects on stopping the course of degenerative disease and reenergizing the bodies own defense mechanisms to bring it into homeostasis (optimum health). Our proven medical protocol includes a 3 phase approach to eradication of disease and restoration of homeostasis. the goal of this protocol is not to "take this pill once a day for the rest of your life". But through breakthrough therapeutics, optimum nutrition and immune surveillance bring the patient to the health once enjoyed and a quality of life reestablished.Immunotherapy and Immunomodulators:
Immunotherapy
Immunotherapy is the treatment of disease by inducing, enhancing or suppressing an Immune Response. Jedd Wolchok MD summed up Immunotherapy just last year in response to Immunotherapy being Science Magazines 2013 Breakthrough of the Year. "10 years ago Immunotherapy was almost in the category of snake oil. A few years ago it was stated that Immunotherapy can work. Now Immunotherapy has entered the main stream." James T. Allison, PhD, professor and Chair of Immunology at the MD Anderson and the Cancer Research Institute (CRI) has received the prestigious Tang Prize, the Louisa Gross Horwitz Prize and the Harvey Prize, all in 2014 for his work on CTLA-4 as an antibody inhibitor in many treatment resistant cancers with amazing survival statistics. Work in CTLA-4 & PD-1 also called "immune check point therapies" are the only two presently approved FDA ImmunoTherapy agents so far.Why Immunotherapy?
Specificity. T-cells recognize peptides, which are short chain biological molecules that are produced by every cell category including virus, bacteria, cancer/mutations. T-cells can recognize them and can destroy them, and most importantly they have the following two important elements.First, Memory. As T-cells destroy pathogens, which are biological or environmental invaders which produce abnormal cells, they go through 3 phases: expansion, contraction and memory.
- Expansion is when the T-cells multiply to overwhelm the attacker, including cancers.
- Contraction is when they diminish due to program cell death after the attacker is neutralized.
- Memory is a multiplication of cells that remember the genetic code of the attacker. Memory cells proliferate and circulate through the body standing ready to attack.
Immunomodulators:
An immunomodulator is a substance that either suppresses or activates the body's immune response. These substances are separated into two groups: immunosuppressants and immune activators. Immunosuppressants inhibit the body's natural immune response, while immune activators generally condition or reprogram it to target a specific disease-causing agent.Immunomodulators can be produced in synthetic form or naturally in the body. Cytokines are examples of innate immune mediators. Synthetic versions are available in either immunosuppressant or immune activator forms. A suppressant immunomodulator works by inhibiting the activation of critical immune system agents such as calcineurin and the formation of thymus cells (T-cells) and antibodies. In comparison, an activating immunomodulator uses the process of adaptive immunity to recondition lymphocytes and T-cells to kill known pathogens or tumor cells.
As an immunomodulator, our active ingredient is one of the few molecules that affects and modulates both the innate and adaptive immune systems without negative side effects, as well as modulating from either activation or suppression immunotherapy depending on what the body needs.
Cyclosporine and methotrexate are commonly used synthetic immunosuppressors. Methotrexate is used in patients with autoimmune ailments. Lupus and rheumatoid arthritis are examples of autoimmune disorders that cause the patient's body to attack his or her own cells. Eventually the targeted cells and tissue become damaged after repeated attacks.The process of organ rejection is similar to autoimmune dysfunction, except the immune system targets the transplanted organ rather than the body's own cells. Organ transplant recipients take suppressant drugs such as cyclosporine, tacrolimus and sirolimus to prevent organ rejection. Nearly all transplant recipients, except a rare few, must adhere to a strict daily regime that involves taking these medications for life. Not taking the medications as prescribed will almost always induce organ rejection, which could lead to death. Due to the medication's toxic side effects, immuno-suppressors should only be used in cases of severe autoimmune dysfunction or organ transplantation. Ethogen's Breakthrough Therapeutic will be investigated in the near future for organ transplant anti-rejection and avoidance graft-versus-post disease (GvHD).
Immunomodulators that activate the immune system include vaccines and cancer immunotherapy. Vaccines work by exposing the patient to weakened or inactive forms of certain bacteria and viruses. The immune system then adapts by producing antibodies that are programmed to immediately kill the introduced pathogen once it re-enters the body, which is called adaptive immunity.
Cancer immunotherapy is very similar to pathogen vaccination. The difference between
the two therapies is the agent in which adaptive immunity is induced. Vaccines use
microorganisms, while cancer immunotherapy uses microorganisms and enhanced
immune cells. Microorganism-based cancer immunotherapies are used to combat some
forms of cervical and liver cancers caused by viruses. A cell-based immunomodulator,
on the other hand, uses enhanced immune cells such as cytotoxic T lymphocytes
(CTLs), dendritic cells (DC) and natural killer cells (NK cells) to target and destroy the
patient's cancerous cells.