Clinical Evidence (Mozart Study)
A safe and effective treatment for pain in moderate to severe knee osteoarthritis
In the Mozart study, combined injections with Traumeel and Zeel provided statistically significant pain relief on days 15 to 99 compared to placebo- The achieved pain relief was clinically relevant (minimal clinically important improvement of -32.6 mm)
- Even after 99 days the percentage of subjects achieving decrease in WOMAC pain subscale score of at least -32.6 mm compared to baseline was significantly higher than in the placebo group
- In this double-blind, randomized, controlled trial, a mineral/biological multi-extract combination was shown to be a safe and effective treatment for pain in moderate to severe knee OA
Mozart Study Design
In this multi-center, double-blind, randomized, controlled trial, 232 patients with moderate-to-severe chronic knee OA were randomized to 3 weekly IA injections of either combined Traumeel & Zeel Injection Solutions or saline by clinical investigators experienced with use of the IA injection route. The primary efficacy variable was change in knee pain from Baseline to End-of-Study (Week 17) as measured by the WOMAC OA Pain Subscale (Section A, 1-5) 100 mm VAS. Secondary measures included Total WOMAC and sub-scores for stiffness (B), and physical function (C), change in pain following a 50 ft. walk (100 mm VAS), patient and physician global assessments.Clinical relevance was assessed by comparing proportions of patients with reductions from baseline in WOMAC A scores greater than a validated benchmark Minimal Clinically Important Difference (MCID). This was chosen as =32.6 mm (the most conservative value) based on a study of outpatients with knee or hip OA where WOMAC VAS MCIDs ranged from=7.9mm to=32.6mm [see Tauback et al., Ann Rheum Dis. 2005; 64(1):29-33 in the description of the WOMAC index published by ACR]. Safety was assessed by monitoring of vital signs, physical examinations of the target knee, adverse events and concomitant medications.
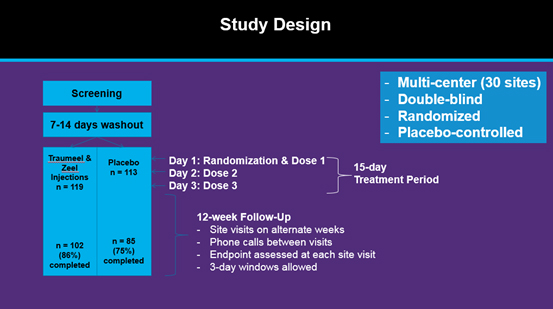
Patient Population
| Co-administered Traumeel® and Zeel® | Placebo Injectable Solution | Total | |
|---|---|---|---|
| Number of Participants | 119 | 113 | 232 |
| Age Mean ± Standard Deviation | 60.7 ± 9.11 | 59.7 ± 8.68 | 60.2 ± 8.90 |
| Gender | |||
| Female | 43 | 46 | 89 |
| Male | 76 | 67 | 143 |
| Region of Enrollment | |||
| United States | 119 | 113 | 232 |
WOMAC Pain Score
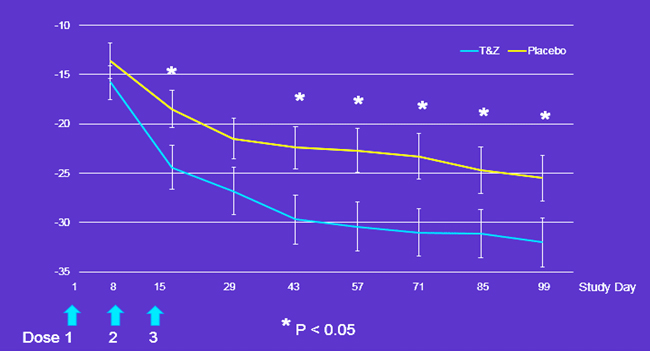 Figure: Mean WOMAC A (Pain) Changes from baseline. Solutions did not discriminate for WOMAC A Pain as expected after only 1 of 3 injections on Day 8 (p=0.3715), but subsequently was significantly different (p=0.05) on Days 15, 43, 57, 71, 85 and 99 (primary endpoint day), and approached significance on Day 29 (p=0.0686).
Figure: Mean WOMAC A (Pain) Changes from baseline. Solutions did not discriminate for WOMAC A Pain as expected after only 1 of 3 injections on Day 8 (p=0.3715), but subsequently was significantly different (p=0.05) on Days 15, 43, 57, 71, 85 and 99 (primary endpoint day), and approached significance on Day 29 (p=0.0686).Clinical Relevant Pain Relief
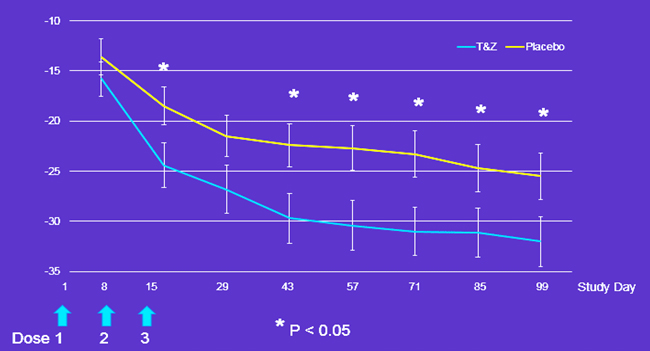 Figure: Percentage of subjects achieving decrease in WOMAC pain subscale score of ≥ 32.6 mm from baseline. ). Logistic regressions showed the proportion of MCID responders was not significant on Day 8. As this was an expected finding, it served as a no-effect internal-model-validation. Traumeel & Zeel Injection Solutions was significantly different (p=0.05) on all subsequent days except Day 29 (approached significance, p=0.0599).
Figure: Percentage of subjects achieving decrease in WOMAC pain subscale score of ≥ 32.6 mm from baseline. ). Logistic regressions showed the proportion of MCID responders was not significant on Day 8. As this was an expected finding, it served as a no-effect internal-model-validation. Traumeel & Zeel Injection Solutions was significantly different (p=0.05) on all subsequent days except Day 29 (approached significance, p=0.0599).
50' Walk Test
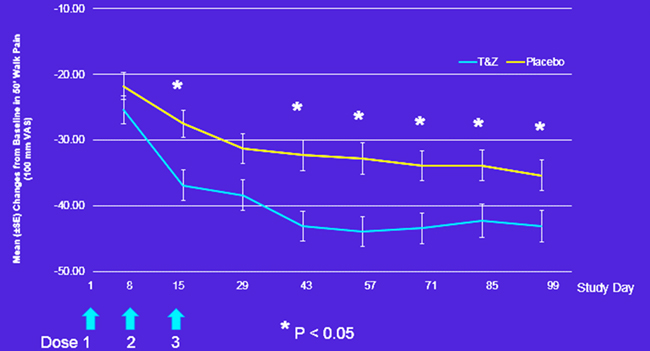 Figure: Mean changes from baseline in 50' walk pain.
Figure: Mean changes from baseline in 50' walk pain.
Footnotes
PDF for download of ACR and EULAR abstract